The COVID-19 Community Research Partnership Celebrates its One-year Anniversary
April 9, 2021
On April 9, 2020, we officially enrolled our first participants. And thanks to all of you, we have learned so much about how to fight COVID-19. Since then...
- We have enrolled 23,861 participants from Wake Forest Baptist Health and over 59,007 through our study partner sites both in North Carolina and nationally.
- We have results for over 61,513 antibody tests from Wake Forest Baptist Health participants and 93,990 results from participants study wide.
- Wake Forest Baptist Health Study participants have completed over 4 million total daily surveys and there have been 6,496,983 daily surveys completed study-wide. Below are some of the participants who have completed the most daily surveys:
- Michael F.
- Melanie H.
- John P.
- Arjun C.
- Maryjo C.
- Jennifer D.
Because of you, we have provided valuable data to the Centers for Disease Control about the prevalence of the COVID-19 antibody as well as the effectiveness of the vaccine within North Carolina and beyond.
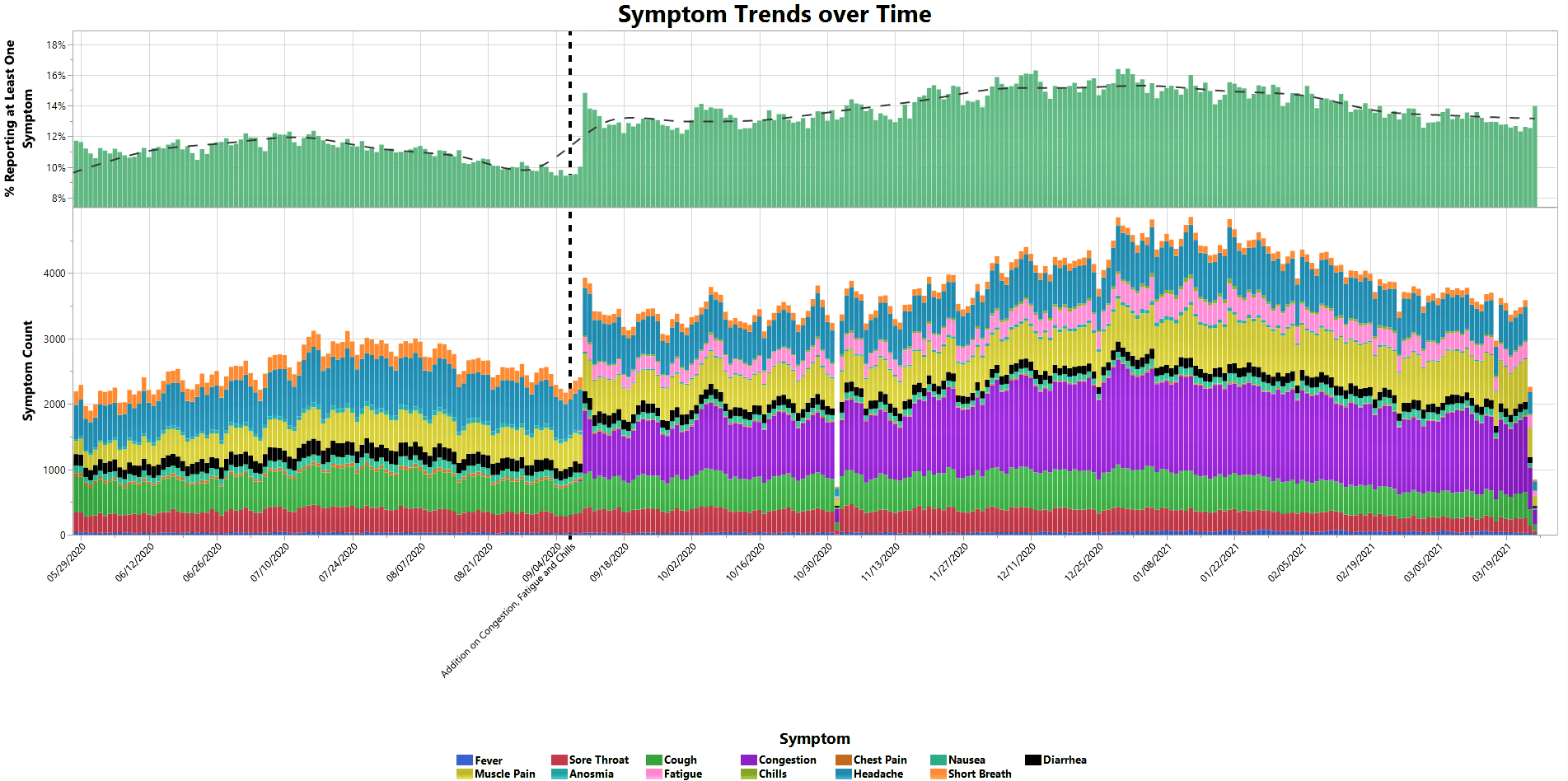
The graphic below shows how many symptoms and the type of symptoms you reported since the beginning of the study. This data helped us to predict how the virus moved through our community.
Total Symptoms
Fever: 12,303
Sore Throat: 101,876
Cough: 163,676
Congestion: 219,329
Confused: 2,742
Chest Pain: 9,425
Nausea: 32,852
Blue Lips: 281
Diarrhea: 49,370
Muscle Pain: 157,224
Anosmia(Loss of Taste/Smell): 11,740
Fatigue: 49,477
Chills: 6,096
Headache: 185,632
Shortness of Breath: 47,474
Thank you for joining the fight against COVID-19!
 Donation Made for Reaching 3.25 Million Updates
Donation Made for Reaching 3.25 Million Updates
 Donation Made for Reaching 3.25 Million Updates
Donation Made for Reaching 3.25 Million Updates
February 9, 2021
A $2,000 donation to The Hispanic League, of Winston-Salem, was made by local non-profit organization Greater Gift on behalf of the participants of the COVID-19 Community Research Partnership. The gift was made to celebrate the milestone of 3.25 million daily symptom updates made by participants who report any symptoms that may be related to COVID-19. More donations will be made for reaching future milestones. Congratulations to all study participants for helping to join the fight against COVID-19.
Donation Made for Reaching 3 Million Updates
February 1, 2021
A $2,000 donation to The Twenty, Inc., of Winston-Salem, Greensboro, and High Point was made by local non-profit organization Greater Gift on behalf of the participants of the COVID-19 Community Research Partnership. The gift was made to celebrate the milestone of 3 million daily symptom updates made by participants who report any symptoms that may be related to COVID-19. More donations will be made for reaching future milestones. Congratulations to all study participants for helping to join the fight against COVID-19.
Get Test Kits for Your Friends and Family!
December 15, 2020
Send this link to your friends and family so they can join the study: https://www.wakehealth.edu/Covid19CommunityStudy
As of December 10, 2020, all current study participants will start receiving antibody testing kits. Antibody testing along with daily symptom tracking are both critical to monitoring the impact that COVID-19 and the new vaccines are having on the pandemic. The study will also provide antibody testing kits to at least the first 8,000 new enrollees in the study.
You should continue to track your symptoms and administer antibody tests after you’ve received a COVID-19 vaccination. New participants can enroll if they’ve already received the COVID-19 vaccine or if they plan to do so. You do not need to be a patient of Wake Forest Baptist Health to participate.
 Donation Made for Reaching 2.25 Million Updates
Donation Made for Reaching 2.25 Million Updates
November 19, 2020
A $2,000 donation was made by Greater Gift to Second Harvest Food Bank of NW NC on behalf of the participants of the COVID-19 Community Research Partnership. Greater Gift is a nonprofit organization committed to increasing awareness of clinical research, especially among underrepresented communities, to improve global health. The gift was made to celebrate 2.25 million daily symptom updates made by participants who report any symptoms that may be related to COVID-19. More donations will be made for reaching future milestones. Congratulations to all study participants for helping to join the fight against COVID-19!
COVID-19 Community Research Partnership Town Hall
September 14, 2020
COVID-19 Community Research Partnership Town Hall
View a recording of our first Town Hall with Dr. John Sanders, one of the study’s principle investigators, held on August 31, 2020.
Researchers Adjust Antibody Tests to Improve Study Data Quality
July 28, 2020
An important part of the research process is making the best decisions possible at each stage and continually making changes as more and better information becomes available. Learning about the novel coronavirus is real-time discovery.
Because COVID-19 antibody tests are relatively new, they must undergo a review process to confirm that they work and that their results are statistically “valid.” Tests are measured by sensitivity and specificity. Sensitivity is a measure of how well a test finds antibodies in someone who has been infected. Specificity is a measure of how accurate the test is at confirming negative results.
The antibody tests we have been using as part of the COVID-19 Community Research Partnership have undergone 3 separate reviews: 1 by Wake Forest Baptist Health, 1 by LabCorp, and 1 by the National Cancer Institute (NCI), at the request of the Food and Drug Administration (FDA).
In the first 2 reviews, the test sensitivity was 90% and test specificity was 100%. This meant that the test identified antibodies in 9 out of 10 samples from patients known to have COVID-19 and did not identify any antibodies in samples that had been collected and saved from before COVID-19 existed (no false positives).
In the third review, test sensitivity showed 93% for one type of antibodies (IgM) and 77% for the other type (IgG), meaning there were a larger number of false negative tests. Test specificity was 97.5% for IgM and 100% for IgG.
Although the test we have been using performed well on the first 2 reviews and the average of the 3 reviews showed overall good performance, the third review raised concerns that the test may not be sensitive enough (too many false negatives). As a result and because we continually want to improve the quality of our data, we have changed our antibody tests.