What You Need to Know About COVID-19
During this challenging time, Atrium Health Wake Forest Baptist is doing everything we can to make sure you stay safe and informed.
COVID-19 Vaccination
Discuss vaccination with your primary care provider. If you need a primary care provider, you can search for one online. If you’re immunocompromised and have questions, see the CDC’s guidance for immunocompromised people for more information.
What to Do If I Feel Sick?
Always listen to your health care provider. It is important that you follow their instructions to keep you, your family and your community safe.
For Mild Illness

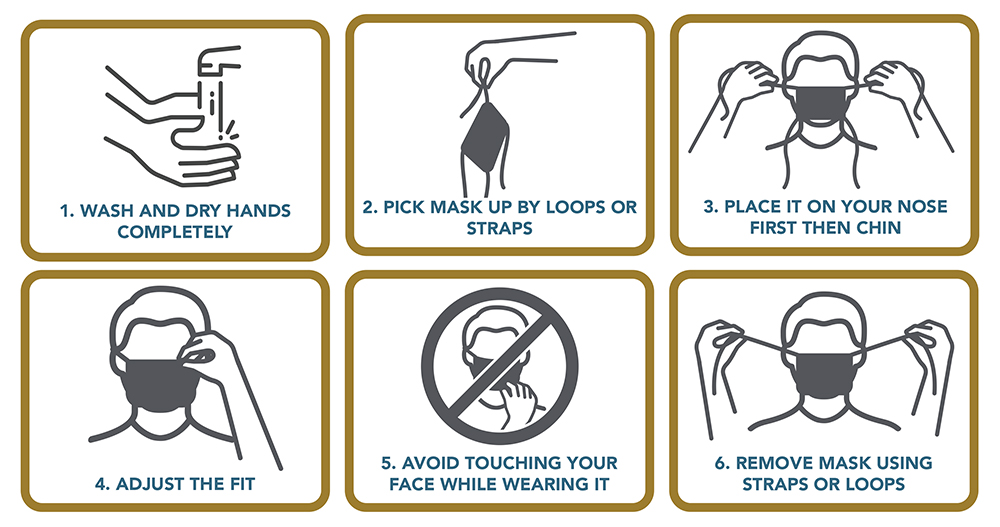
Stay home and distance yourself from others. How to practice safe hygiene and keep yourself isolated.
If Your Illness Worsens

Call your primary care provider. If you do not have a primary care provider, call 336-716-WAKE for assistance.
More care options, including telehealth services.
How to Get Tested

Call your primary care provider or go to a free community testing site. Only go to the ED or call 911 if you are experiencing a life-threatening emergency. More testing information.
Symptoms of COVID-19
People with COVID-19 have had a wide range of symptoms reported – ranging from mild symptoms to severe illness. Symptoms may appear 2-14 days after exposure to the virus. Anyone can have mild to severe symptoms.
Pricing of COVID-19 Diagnostic Testing
During the COVID-19 emergency period declared by the Public Health Act, providers are required to make public the cash prices for the diagnostic test for COVID-19 [Reference: CARES Act Sec. 3202]. The cash price shown is for Atrium Health Wake Forest Baptist entities in all care settings where tests are performed.
*COVID-19 Diagnostic Test Cash Price: $125
We Promise to Keep You Safe, Healthy and COVID-Protected
Following advice from our infectious disease experts, we will be:
- Practicing social distancing in all of our clinic locations
- Minimizing wait times in common areas, like waiting rooms
- Asking our patients to join all providers and clinic staff in wearing masks
- Limiting visitors, with few exceptions
- Cleaning our facilities with strict sanitation protocols
- Conducting screenings for COVID-19 symptoms upon arrival
- Offering expanded access to virtual visits via video or telephone
COVID-19 How You Can Help
As the impact of the novel coronavirus (COVID-19) continues to develop, providers, researchers and staff from across Atrium Health Wake Forest Baptist are working diligently to provide for you and your family.
Many people have asked how they can best support our system during this challenging time. Given the strain on our resources to address this situation, your support is needed and appreciated.
More Resources
-
COVID-19 Vaccine Study
You can help researchers study an investigational vaccine to potentially prevent COVID-19 by volunteering in this clinical trial.
-
Stress and Coping
Coping with stress during a COVID-19 outbreak will make you, your loved ones, and your community stronger.
-
COVID-19 Resources for Pediatric Providers
The pediatric experts at Brenner Children's recommend several resources to rely on during the ever-changing the COVID-19 pandemic.
Weekly COVID-19 Update with Dr. Christopher Ohl
We were live with Christopher Ohl, MD, infectious disease expert, as he provided an update on COVID-19 in our region.
Like Atrium Health Wake Forest Baptist on Facebook to stay up to date with the latest COVID-19 information.
Disclaimer: Learn more about the latest developments with visitor restrictions and testing.